Program Overview
This program follows a structured curriculum that is delivered over 2 years. Starting July 2, 2024, a new educational offering will be posted weekly. All learning modules are open for public access, offering a great opportunity for everyone interested in learning about pragmatic clinical trials to do that at their own pace, in a virtual and asynchronous setting.
More than 75 learners have been admitted to the program after a competitive selection process. The learners formally participating in the program will benefit from monthly facilitated group sessions, 2 annual conferences, as well as the opportunity to work with a diverse group of researchers, professors, clinicians, and health care workers who come with various specialties, backgrounds, and professional experiences.
Interested learners who have not joined the 2-year structured program can still benefit from the program’s weekly-updated and publicly-accessible learning modules.
Applications to formally join the program are now closed.
Curriculum
The curriculum for this training program contains 12 interrelated sections. Given that the design, implementation, and management of pragmatic trials is a non-linear process, featured modules will not follow a specific order. Instead, modules will relate to various sections of the curriculum wheel over time.
The curriculum and its 12 sections are the following:
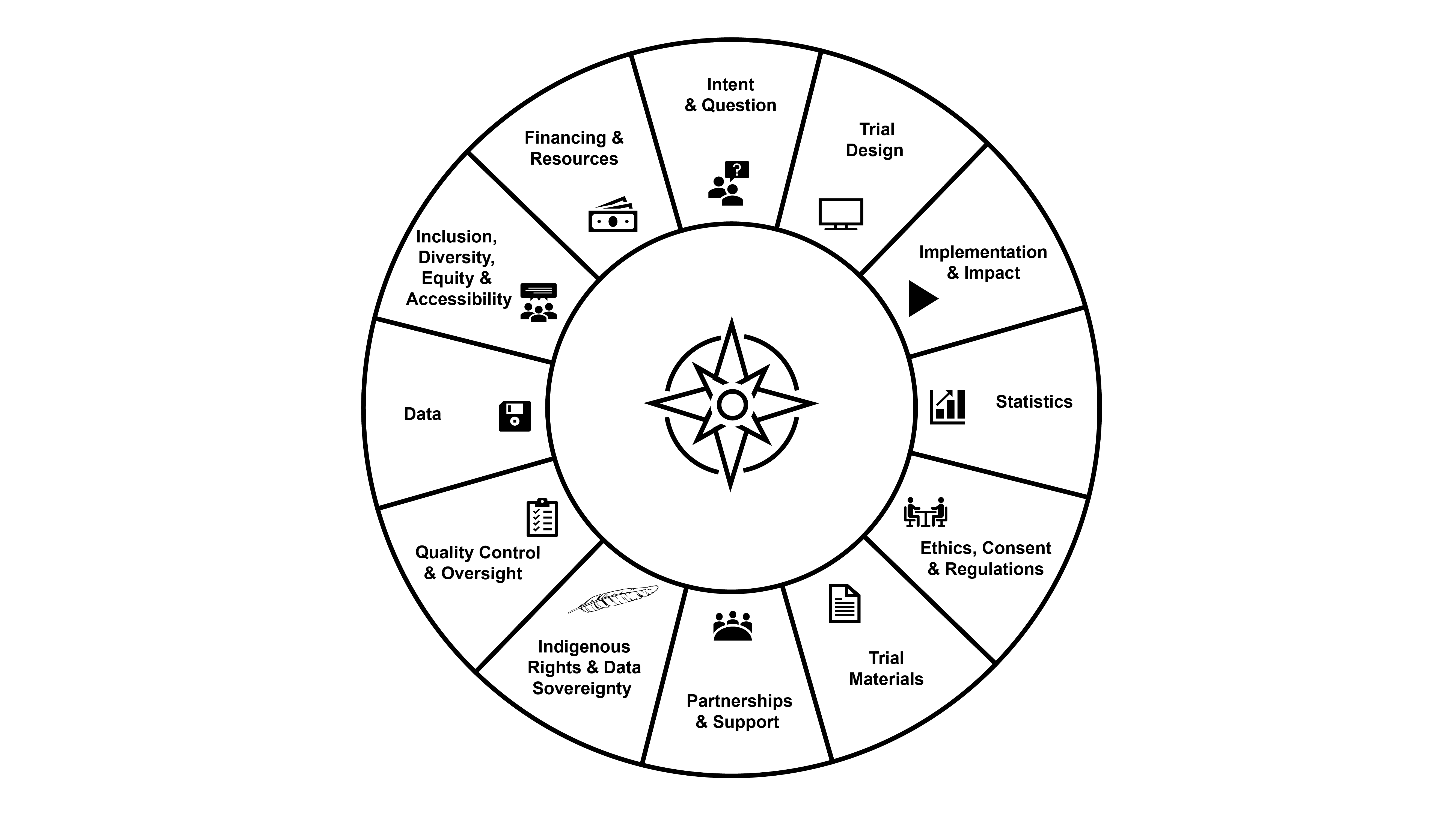
As modules from the different sections are featured, they will be posted on the educational website.
Program duration: July 2, 2024 - June 30, 2026 (2 years)
Additional Information
In addition to asynchronous education, the program offers learners the following:
- Facilitated group sessions: These virtual peer-group sessions, involving 6 - 10 people per group, will be held monthly for an hour over the upcoming two years. Each session will be moderated by an experienced facilitator who will encourage robust discussions.
- Annual virtual conference: The first 4-day conference will be held March 3 to 6, 2025.
- Indigenous knowledge: Participants will have access to specialized training that aims to enhance their knowledge and awareness of racial biases, Indigenous voices and stories, the impact of colonization on Indigenous health, and culturally safe health research practices.
For more, check our infographic.








