Study validates new biopsy method for breast cancer patients
By Lawson Institute Communications
A study in the American Journal of Roentgenology, authored by a team at Lawson Health Research Institute, is the first in North America to find that a new breast cancer biopsy method may offer a more accurate and comfortable option for patients.
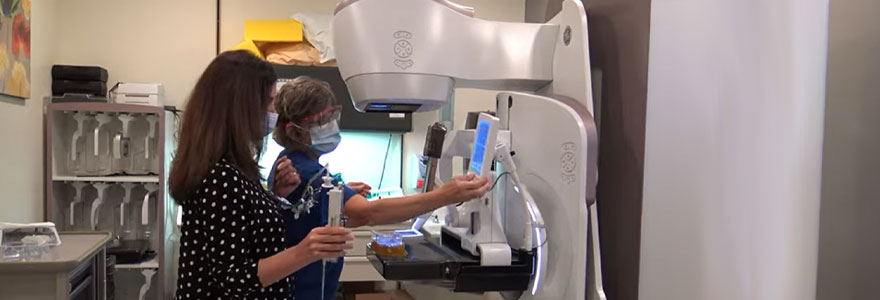
The method is a new form of mammography software that combines contrast enhanced mammography (CEM) with mammography guided biopsy technology at St. Joseph’s Health Care London’s Breast Care Program. These tools were combined in an effort to make the biopsy procedure more streamlined, accurate, and easier for patients and technicians.
CEM is a relatively new form of mammography that uses contrast iodine injected intravenously, which acts like a dye that allows radiologists to spot potential cancerous lesions more effectively. If potential lesions are found, a biopsy is often the next step.
 Dr. Anat Kornecki, Medical Imaging professor, Schulich School of Medicine & Dentistry (photo supplied)
Dr. Anat Kornecki, Medical Imaging professor, Schulich School of Medicine & Dentistry (photo supplied)
CEM is a relatively new form of mammography that uses contrast iodine injected intravenously, which acts like a dye that allows radiologists to spot potential cancerous lesions more effectively. If potential lesions are found, a biopsy is often the next step.
Before this option was made available to patients through this research, suspicious lesion detection that was only seen on contrast enhanced mammography were biopsied under MRI. This meant longer procedures and working with limited MRI availability.
“If a lesion is detected only by CEM we usually offer an MRI guided biopsy, but we first need to find the same lesion on an MRI,” said Dr. Anat Kornecki, Lawson associate scientist and breast radiologist lead at St. Joseph’s Health Care London. “The problem is that it is sometimes hard to find the same lesion and the MRI itself can be uncomfortable for the patient. Also, some lesions that are close to implants or chest walls cannot be reached with MRI guided biopsy.”
Kornecki and her research team therefore decided to study this new method. They were the first in North America to trial CESM-guided biopsies by using new technology created by GE HeathCare. This software means that patients can have the biopsy done with the exact same modality, avoiding the need for an MRI.
The study included 50 patients through St. Joseph’s Breast Care Program. The research team found 51 potentially cancerous breast lesions. Biopsies were successfully performed for 46 of the lesions. The results showed that 11 were breast cancer, 10 were high-risk lesions, and the remaining were benign lesions.
“These are very similar results that were reported through MRI-guided biopsies, which means that this new method can replace the MRI,” explained Kornecki.
Patients also reported having a more comfortable experience with the CEM-guided biopsy method.
Researchers in London and at two other centres in Europe were the first to pilot this technique which has now been cleared by Health Canada and the FDA commercially. St. Joseph’s Breast Care Program was the first site in North America to offer this procedure as a clinical standard of care.
“It is a game changer with certainty,” added Kornecki. “This is now a great added component for patients, which makes it a very good tool.”
Currently CEM-guided biopsies can be offered to patients with lesions that were initially detected by MRI where a biopsy is not feasible due to the lesion location. While it is currently being used as a diagnostic tool only, Kornecki is hopeful that eventually CEM-guided biopsies will be approved as an initial breast cancer screening tool as well.









