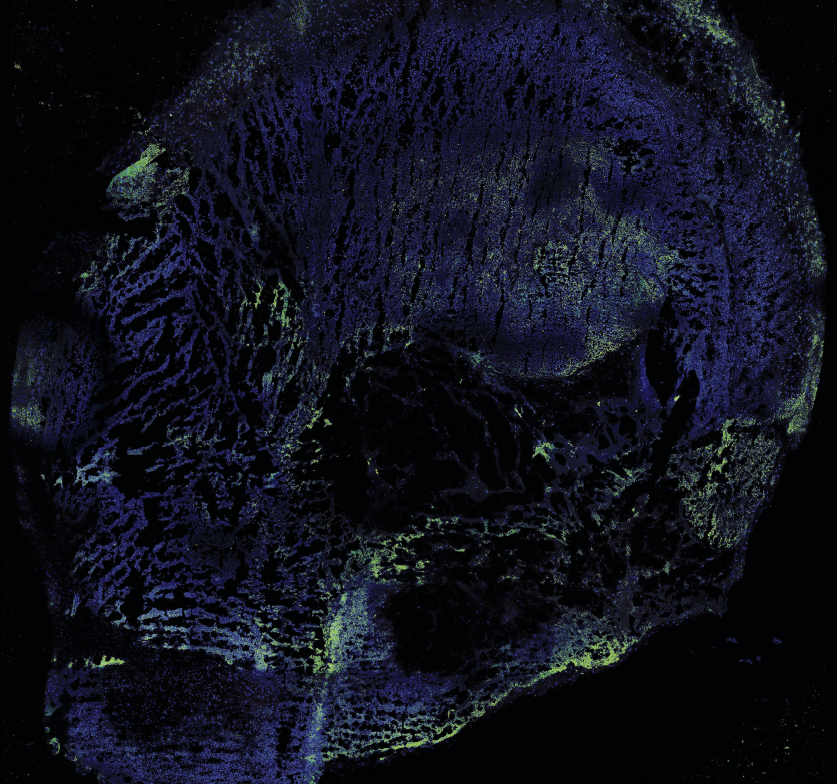
 Image of a human intestinal organoid, showing the various cell types in different colours.
Image of a human intestinal organoid, showing the various cell types in different colours.
Image supplied by Van Lu
In 2009, Dutch molecular geneticist Hans Clevers suspended human stem cells from the small intestine in “bio gel” to provide them with structural support and room to grow.
And something incredible happened.
The cells started to differentiate and self-organize to form organoids. These three-dimensional miniature versions of organs revolutionize the way researchers study disease development and possible treatments.
Like others around the world, Schulich Medicine & Dentistry researchers are using these revolutionary organoids to advance their research into psychiatric disorders, including addiction, pancreatic cancer and understanding the mechanisms of the gut.
 “These organoids allow us to model the earliest periods of human brain development in a dish and give us access to the entire genetic background of these patients.” —Steven Laviolette, PhD
“These organoids allow us to model the earliest periods of human brain development in a dish and give us access to the entire genetic background of these patients.” —Steven Laviolette, PhD
Modelling the human brain
Professor Steven Laviolette, PhD, Department of Anatomy and Cell Biology and Department of Psychiatry, uses human brain organoids in his research to examine the neurobiological and molecular mechanisms underlying various psychiatric disorders, such as addiction and schizophrenia.
What’s exciting about using organoids is that human psychiatric disorders can be modelled from real human samples, said Laviolette. His lab is particularly interested in the impacts of prenatal drugs, like nicotine and cannabis, on the developing brain and how that sets people up for increased psychiatric risk later in life.
Laviolette’s lab focuses on cells derived from schizophrenia patients.
“These organoids allow us to model the earliest periods of human brain development in a dish and give us access to the entire genetic background of these patients,” he said.
The work with organoids allows researchers to draw stronger conclusions from their work, while avoiding the ethical problems and practical limitations of clinical research in human populations.
For example, clinical evidence suggests children of mothers who smoked heavily during pregnancy have an increased risk of developing schizophrenia. Using this model, they can safely look at the impacts in healthy individuals versus individuals who have a genetic risk for developing schizophrenia.
Laviolette and his team are also currently using organoids to better understand the relationship between delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in cannabis.
THC is the main psychoactive compound in cannabis and believed to lead to dependence and increased risk for various psychiatric disorders, such as schizophrenia, while CBD has been shown in Laviolette’s earlier research to prevent a lot of the negative effects of THC.
Laviolette’s lab is exposing organoids to different levels of THC, CBD and combinations of both. The data is still being analyzed, but so far it suggests CBD might mitigate some of the negative impacts of THC on early brain development, consistent with previous animal research studies, said Laviolette.
 “Using organoids to understand how different components interact will provide better insights into why some patients don’t respond to a certain treatment and what can be done to change the response.” —Christopher Pin, BSC Honors’90, PhD’95
“Using organoids to understand how different components interact will provide better insights into why some patients don’t respond to a certain treatment and what can be done to change the response.” —Christopher Pin, BSC Honors’90, PhD’95
Laviolette’s brain organoid research extends to looking at potential treatment interventions. “What’s exciting about the new technology, is it allows for testing of an unlimited number of potential treatment interventions without putting patients at risk,” he said.
The key to precision medicine
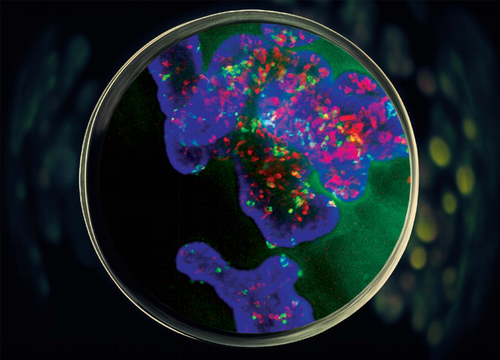
Christopher Pin, BSC Honors’90, PhD’95, and professor of Physiology and Pharmacology, is the lead of a Western University interdisciplinary initiative grant that focuses on organoid research. His research is centred on finding therapies to treat pancreatic cancer and he echoed Laviolette’s excitement for the new organoid technology.
Pin explained that 85 per cent of people with cancer of the pancreas have pancreatic ductal adenocarcinoma, a very deadly type of cancer. This kind of cancer has many forms that respond to treatment very differently from patient to patient.
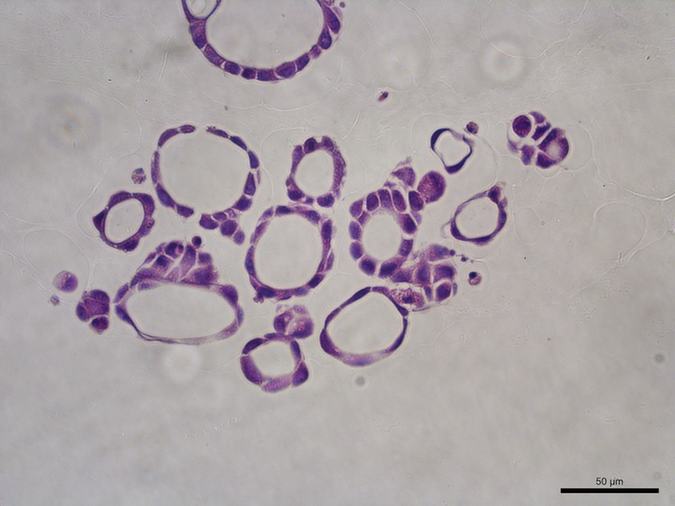
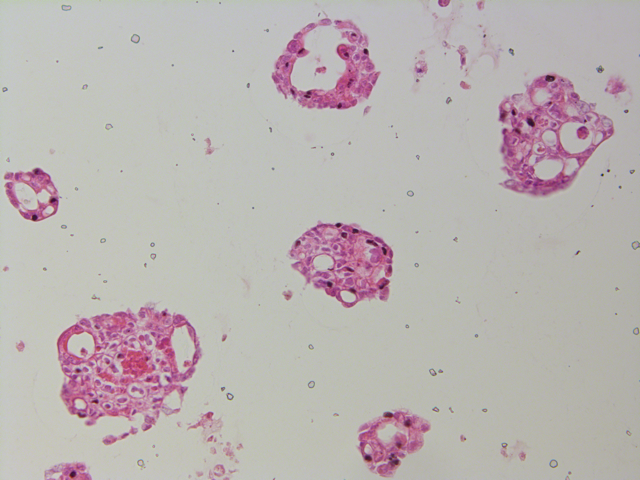
Images supplied by Christopher Pin
However, researchers can do genomic analysis and look at the gene expression, which may give them new targets for treating patients, said Pin. “It’s a very exciting time.”
According to Pin, his lab is the only one in Canada that uses tissue samples obtained via endoscopy from patients with suspected pancreatic cancer. This approach allows researchers to quickly grow pancreatic cancer organoids specific to the individual patient.
Developing personalized treatment options using pancreatic cancer organoids is still in their infancy. However, the hope is that researchers can see how the pancreatic cancer organoid responds to the standard treatment and also try new or combination approaches for patient-specific treatment, said Pin. If an effective treatment is found, it may be a potential therapy that will work specifically for the patient.
What is equally important are the non-cancerous cells that surround the tumour, because the way these cells communicate and interact with cancer cells can affect the response to treatment, said Pin. There is also the immune component to consider. Using organoids to understand how these different components interact will provide better insights into why some patients don’t respond to a certain treatment and what can be done to change the response.
Understanding disease development
 “Human organoids are great models because they introduce complexity and more resemble what occurs in a human.” —Van B. Lu, PhD
“Human organoids are great models because they introduce complexity and more resemble what occurs in a human.” —Van B. Lu, PhD
Van B. Lu, PhD, assistant professor in the Department of Physiology and Pharmacology, grows human organoids from different sections of the gastrointestinal tract, such as the upper small intestine and colon.
What makes organoids remarkable is the way they maintain the tissue difference of the specific region of the GI tract.
Both videos supplied by Van Lu
“They don’t just reconvert to a premature cell type; they actually maintain some of the features of the original tissue where they come from,” said Lu, who is also a scientist at Children’s Health Research Institute, a program of Lawson Health Research Institute. This ability to mimic the GI tract allows researchers to compare how different regions function.
Lu is interested in how gastrointestinal epithelial cells respond to dietary nutrients and gut bacteria. She specifically looks at the detailed mechanisms and specific signalling pathways that contribute to this symbiotic relationship. Understanding these cell mechanisms is the first step to understanding disease processes, such as inflammatory bowel disease (IBD) and how the gut microbiome influences human health. Human organoids are crucial to modelling these different mechanisms, as the gut bacteria in animal or rodent models are very different from human gut bacteria.
Lu’s team studied the different gut mechanisms in mice, but when they asked if these mechanisms occurred in the same cell type in humans, the answer was quite different, said Lu. Lu and her team do a lot of genetic editing, such as inserting a fluorescent gene into the organoids using a gene-editing technology called CRISPR/Cas9. This lets them identify cells and monitor cell activity. It can take up to six months to generate genetically altered lines. “It takes a lot of work but the benefit is once you have your population of cells labelled, you can keep maintaining it in culture and have lots of supply,” Lu said.
Organoids are used to model human mechanisms and disease development. Soon, their uses could lead to treatment interventions for diseases or an understanding of a better way to stay healthy.
“Human organoids are great models because they introduce complexity and more resemble what occurs in a human,” said Lu.